Heart diseases can be primarily grouped into three major disorders: cardiac failure, ischemia and cardiac arrhythmia.
Cardiac failure can be described as the inability of the heart to pump blood effectively at a rate that meets the needs of the metabolizing tissues. This occurs when the muscles that perform contraction and force the blood out of heart are
performing weakly. Thus cardiac failures primarily arise from the reduced contractility of heart muscles, especially the ventricles. Reduced contraction of heart leads to reduced heart output but new blood keeps coming in resulting in the increase in heart blood volume. The heart feels congested. Hence the term congestive heart failure.
Congested heart leads to lowered blood pressure and poor renal blood flow. This results in the development of edema in the lower extremities and the lung (pulmonary edema) as well as renal failure.
Increasing the force of contraction of the heart (positive inotropic activity) is very important for most heart failure patients. There are several mechanisms by which this could be achieved. Cardiac steroids are perhaps the most useful and are being discussed here. Phosphodiesterase inhibitors, such as amrinone and milrinone, have also been explored and so are direct adenylate cyclase stimulants, such as forskolin. These drugs all act by affecting the availability of intracellular Ca+2 for myocardial contraction or increasing the sensitivity of myocardial contractile proteins.
The cardiac glycosides are an important class of naturally occurring drugs whose actions include both beneficial and toxic effects on the heart. Plants containing cardiac steroids have been used as poisons and heart drugs at least since 1500 B.C. Throughout history these plants or their extracts have been variously used as arrow poisons, emetics, diuretics, and heart tonics. Cardiac steroids are widely used in the modern treatment of congestive heart failure and for treatment of atrial fibrillation and flutter. Yet their toxicity remains a serious problem.
Cardiac glycosides are composed of two structural features : the sugar (glycoside) and the non-sugar (aglycone - steroid) moieties. (figure below)

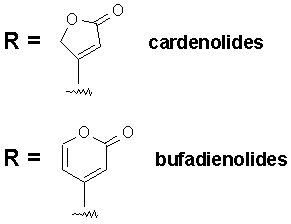
The R group at the 17-position defines the class of cardiac glycoside. Two classes have been observed in Nature - the cardenolides and the bufadienolides (see figure below). The cardenolides have an unsaturated butyrolactone ring while the bufadienolides have an a-pyrone ring.
Nomenclature : The cardiac glycosides occur mainly in plants from which the names have been derived. Digitalis purpurea, Digitalis lanata, Strophanthus grtus, and Strophanthus kombe are the major sources of the cardiac glycosides. The term 'genin' at the end refers to only the aglycone portion (without the sugar). Thus the word digitoxin refers to a agent consisting of digitoxigenin (aglycone) and sugar moieties (three). The aglycone portion (figure below) of cardiac glycosides is more important than the glycone portion.
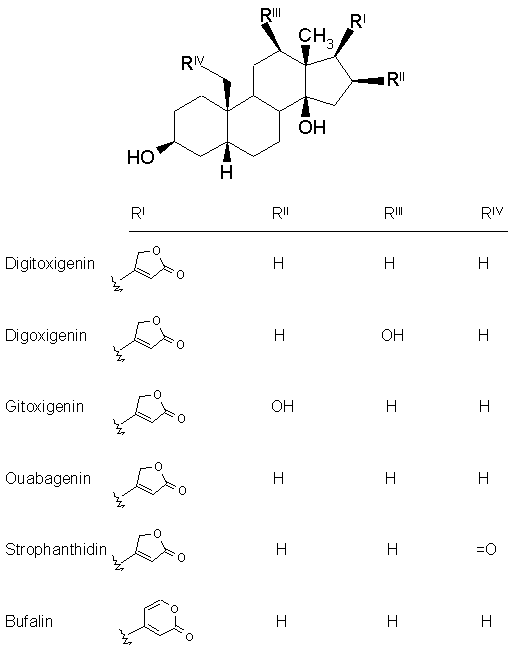
Click the following links to view the three dimensional structures of digitoxigenin,digoxigenin, gitoxigenin, strophanthidin and bufalin. Let us discuss some of the important characteristics of each structural feature.
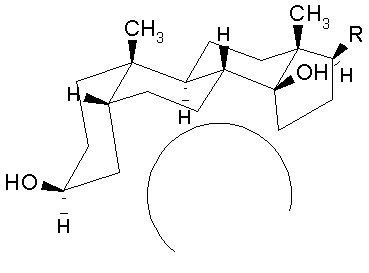
The aglycone moiety: The steroid nucleus has a unique set of fused ring system that makes the aglycone moiety structurally distinct from the other more common steroid ring systems. Rings A/B and C/D are cis fused while rings B/C are trans fused. Such ring fusion give the aglycone nucleus of cardiac glycosides the characteristic 'U' shape as shown below. To view the 3-dimensional structure of the aglycone moiety click on the figure.
The steroid nucleus has hydroxyls at 3- and 14- positions of which the sugar attachment uses the 3-OH group. 14-OH is normally unsubstituted. Many genins have OH groups at 12- and 16- positions. These additional hydroxyl groups influence the partitioning of the cardiac glycosides into the aqueous media and greatly affect the duration of action.
The lactone moiety at C-17 position is an important structural feature. The size and degree of unsaturation varies with the source of the glycoside. Normally plant sources provide a 5-membered unsaturated lactone while animal sources give a 6-membered unsaturated lactone.
Sugar moiety : One to 4 sugars are found to be present in most cardiac glycosides attached to the 3b-OH group. The sugars most commonly used include L-rhamnose, D-glucose, D-digitoxose, D-digitalose, D-digginose, D-sarmentose, L-vallarose, and D-fructose. These sugars predominantly exist in the cardiac glycosides in the b-conformation. The presence of acetyl group on the sugar affects the lipophilic character and the kinetics of the entire glycoside. Because the order of sugars appears to have little to do with biological activity Nature has synthesized a repertoire of numerous cardiac glycosides with differing sugar skeleton but relatively few aglycone structures.
- The sugar moiety appears to be important only for the partitioning and kinetics of action. (see section on pharmacokinetics of cardiac glycosides) It possesses no biological activity. For example, elimination of the aglycone moiety eliminates the activity of alleviating symptoms associated with cardiac failure.
- The "backbone" U shape of the steroid nucleus appears to be very important. Structures with C/D trans fusion are inactive.
- Conversion to A/B trans system leads to a marked drop in activity. Thus although not mandatory A/B cis fusion is important.
- The 14b-OH groups is now believed to be dispensible. A skeleton without 14b-OH group but retaining the C/D cis ring fusion was found to retain activity.
- Lactones alone, when not attached to the steroid skeleton, are not active. Thus the activity rests in the steroid skeleton.
- The unsaturated 17-lactone plays an important role in receptor binding. Saturation of the lactone ring dramatically reduced the biological activity.
- The lactone ring is not absolutely required. For example, using a,b-unsaturated nitrile (C=C-CN group) the lactone could be replaced with little or no loss in biological activity.
The commercially available cardiac steroids differ markedly in their degree of absorption, half-life, and the time to maximal effect (see table below).
| Agent | GI absorption | Onset (m) | Peak (h) | Half-life |
| Ouabain | Unreliable | 5-10 | 0.5-2 | 21 h |
| Deslanoside | Unreliable | 10-30 | 1-2 | 33 h |
| Digoxin | 55-75% | 15-30 | 1.5-5 | 36 h |
| Digitoxin | 90-100% | 25-120 | 4-12 | 4-6 days |
Usually this is due to the polarity differences caused by the number of sugars at C-3 and the presence of additional hydroxyls on the cardenolide. Although two cardiac glycosides may differ by only one sugar residue their partition co-efficients may be significantly different resulting in different pharmacokinetics. For example, lanatoside C and digoxin differ only by a glucose residue and yet the partition co-efficient measured in CHCl3/16% aqueous MeOH are 16.2 and 81.5, respectively.
| Glycoside | Partition Coefficient |
| Lanatoside C (glucose-3-acetyldigitoxose-digitoxose2-digoxigenin) | 16.2 |
| Digoxin (digitoxose3-digoxigenin) | 81.5 |
| Digitoxin (digitoxose3-digitoxigenin) | 96.5 |
| Acetyldigoxin (3-acetyldigitoxose-digitoxose2-digoxigenin) | 98.0 |
| G-Strophanthin (rhamnose-ouabagein) | very low |
In general, cardiac glycosides with more lipophilic character are absorbed faster and exhibit longer duration of action as a result of slower urinary exretion rate. Lipophilicity is markely influenced by the number of sugar residues and the number of hydroxyl groups on the aglycone part of the glycoside. Comparison of digitoxin and digoxin structures reveals that they differ only by an extra OH group in digoxin at C-12, yet their partition coefficients differ by as much as 15 % points.
The mechanism whereby cardiac glycosides cause a positive inotropic effect and electrophysiologic changes is still not completely clear. Several mechanisms have been proposed, but the most widely accepted involves the ability of cardiac glycosides to inhibit the membrane bound Na+-K+-ATPase pump responsible for Na+-K+ exchange.
The process of muscle contraction can be pictured as shown below.
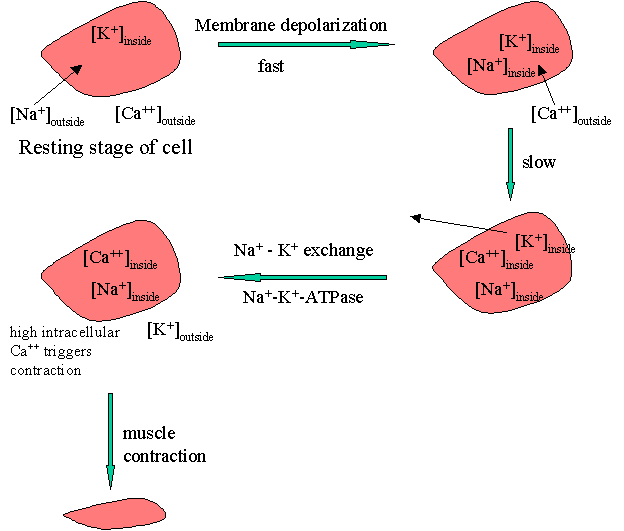
The process of membrane depolarization / repolarization is controlled by the movement of three cations, Na+, Ca+2, and K+, in and out of the cell. At the resting stage, the concentration of Na+ is high on the outside. On membrane depolarization sodium fluxes-in leading to an immediate elevation of the action potential. Elevated intracellular Na+ triggers the influx of free of Ca++that occurs more slowly. The higher intracellular [Ca++] results in the efflux of K+. The reestablishment of the action potential occurs later by the reverse of the Na+-K+ exchange.
The Na+ / K+ exchange requires energy which is provided by an enzyme Na+-K+-ATPase. Cardiac glycosides are proposed to inhibit this enzyme with a net result of reduced sodium exchange with potassium that leaves increased intracellular Na+. This results in increased intracellular [Ca++]. Elevated intracellular calcium concentration triggers a series of intracellular biochemical events that ultimately result in an increase in the force of the myocardial contraction or a positive inotropic effect.
3 comments:
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
warna cat rumah 2019
Thanks for sharing the great information about cardiac disease. I always suggesting the Ayurvedic medicine for heart which is more beneficial and there is no side effects also. heart tablets
I started on COPD Herbal treatment from Ultimate Life Clinic, the treatment worked incredibly for my lungs condition. I used the herbal treatment for almost 4 months, it reversed my COPD. My severe shortness of breath, dry cough, chest tightness gradually disappeared. Reach Ultimate Life Clinic via their website www.ultimatelifeclinic.com . I can breath much better and It feels comfortable!
Post a Comment